Talking yesterday with Chris James of Medsafe at the NZMCC day, we were going over some of the proposed changes to the Medicinal Cannabis Scheme. This included one which I had forgotten to include in the submission response last month: LOD levels. Granted it may be too late now to formally include this, however it's probably worth sending through anyway. If this could please be passed on to him also, I don't have his direct email address.
The current NZMQS refers to European Pharmacopoeia 10th Edition section 2.2.32, with a LOD < 10%. However, cannabis (dried flower) breaks down significantly when it reaches aw levels < 0.55, causing immediate and irreversible damage to the terpenes.
Instead, Chill Division proposes we adopt the standards set out in ASTM D8196 for the Determination of Water Activity in Cannabis Flower, as well as ASTM D8197 for the Standard Specification for Maintaining Acceptable Water Activity Range (0.55 to 0.65) for Dry Cannabis FlowerFor other products such as tinctures, it may be acceptable / more suitable to retain the existing <10% LOD, as the smoke point for MCT oil is approx 160C. As such, in 2.2.32 tests at 105C it will not evaporate.
In order to meet the required levels of <10% LOD, dried cannabis flower medicines have to be what is largely considered "significantly over-dried". This means that when the medicine arrives to the patient, it can often already have significant portions of it turned into dust from this over-drying requirement.There are also adverse implications for patients inhaling through vaporizing, as this is more likely to cause a strong cough. We note that this is likely why Medleaf have included Boveda bags to rehydrate dried flower with some of their medicines, however the (irreversible) damage to the terpenes has already been done, and the breakdown of some trichomes occurs by bringing the product under 0.55aw.
With this in mind still, we must maintain patient safety, and we can use the following chart from the United States Pharmacopoeia 1112 as an example of aw levels at which we will see microbial growth:
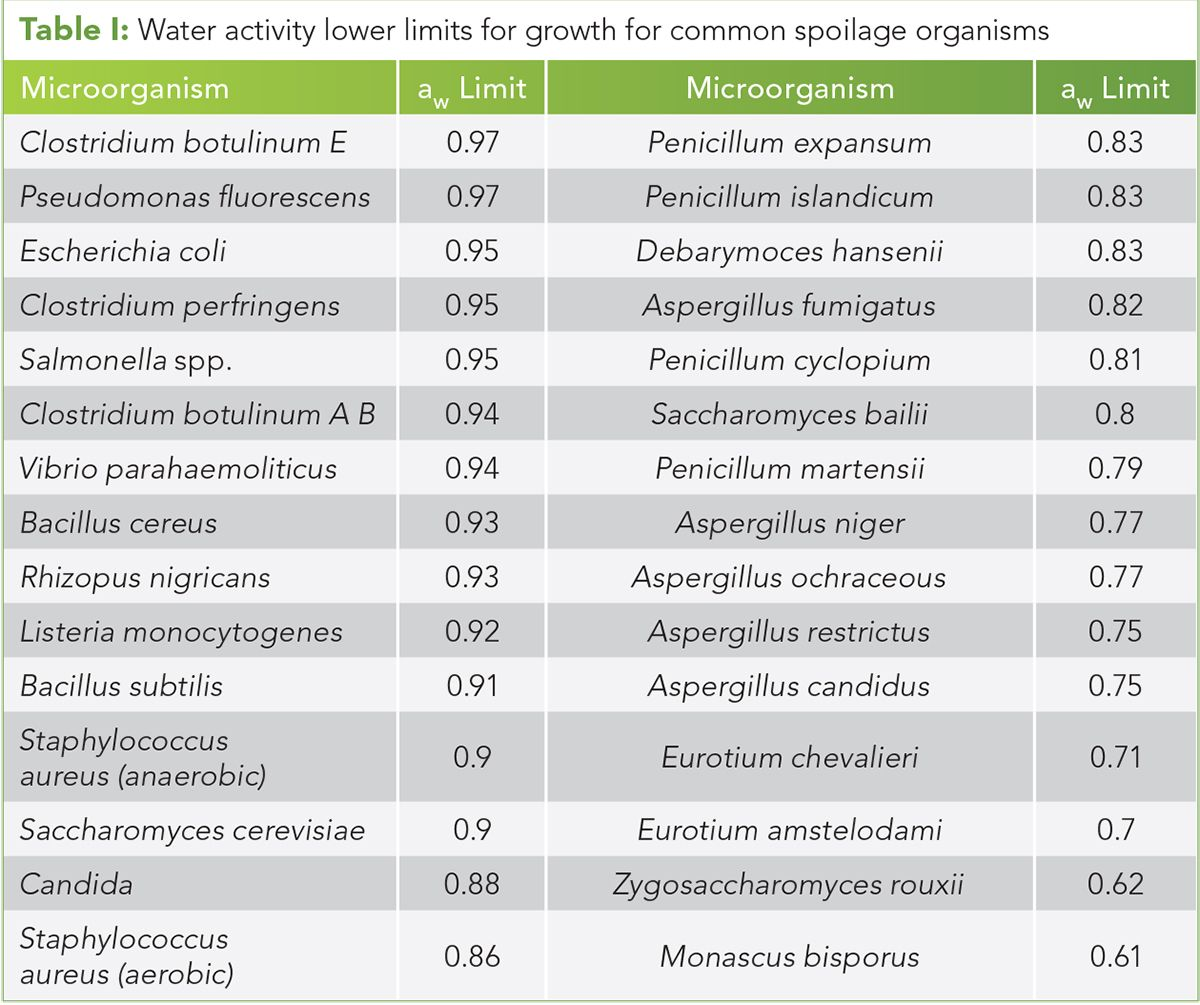
In addition to adopting a more "fit for purpose" set of levels for dried flower products set out in ASTM D8197, by utilising measurement of aw instead of LOD, we will see significantly greater accuracy from permitting accessible, fast, and effective testing of aw levels.
By permitting the levels for dried flower products of 0.55 -> 0.65, this will not only provide a safe range whereby no new microbial growth will occur, it will also prevent physical damage (breakage) during routine handling / shipping. The ASTM D8197 standards also allow for sufficient variances in drying / curing / packaging, but more importantly it will also provide patients with the best possible product.
Again I'd like to thank the Medicinal Cannabis Agency team for receiving this feedback, and to Chris James for taking the time to talk to us all yesterday.
Ngā mihi nui
Josiah
Chill Division